The noncovalent dimerization of a G-quadruplex/hemin DNAzyme improves its biocatalytic properties†
Abstract
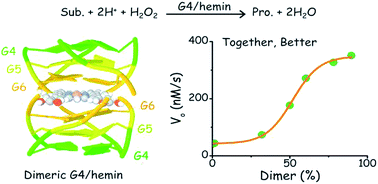
While many protein enzymes exert their functions through multimerization, which improves both selectivity and activity, this has not yet been demonstrated for other naturally occurring catalysts. Here, we report a multimerization effect applied to catalytic DNAs (or DNAzymes) and demonstrate that the enzymatic efficiency of G-quadruplexes (GQs) in interaction with the hemin cofactor is remarkably enhanced by homodimerization. The resulting non-covalent dimeric GQ–DNAzyme system provides hemin with a structurally defined active site in which both the cofactor (hemin) and the oxidant (H2O2) are activated. This new biocatalytic system efficiently performs peroxidase- and peroxygenase-type biotransformations of a broad range of substrates, thus providing new perspectives for biotechnological application of GQs.


 Please wait while we load your content...
Please wait while we load your content...