Abstract
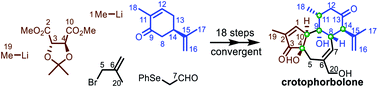
As a natural diterpenoid, crotophorbolone possesses a challenging trans,trans-5/7/6 framework decorated with six contiguous stereogenic centers and is structurally and biogenetically related to tigliane-type diterpenoids with intriguing bioactivities such as phorbol and prostratin. Based on the convergent strategy, we completed an eighteen-step total synthesis of crotophorbolone starting from (−)-carvone and (+)-dimethyl-2,3-O-isopropylidene-L-tartrate. The key elements of the synthesis involve expedient installation of the six-membered ring and the five-membered ring with multiple functional groups at an early stage, cyclization of the seven-membered ring through alkenylation of the ketone between the five-membered ring and the six-membered ring, functional group-sensitive ring-closing metathesis and final selective introduction of hydroxyls at C20 and C4.


 Please wait while we load your content...
Please wait while we load your content...