Visible light driven deuteration of formyl C–H and hydridic C(sp3)–H bonds in feedstock chemicals and pharmaceutical molecules†
Abstract
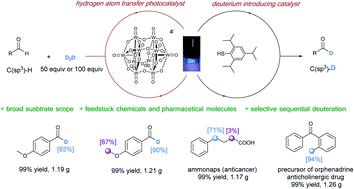
Deuterium labelled compounds are of significant importance in chemical mechanism investigations, mass spectrometric studies, diagnoses of drug metabolisms, and pharmaceutical discovery. Herein, we report an efficient hydrogen deuterium exchange reaction using deuterium oxide (D2O) as the deuterium source, enabled by merging a tetra-n-butylammonium decatungstate (TBADT) hydrogen atom transfer photocatalyst and a thiol catalyst under light irradiation at 390 nm. This deuteration protocol is effective with formyl C–H bonds and a wide range of hydridic C(sp3)–H bonds (e.g. α-oxy, α-thioxy, α-amino, benzylic, and unactivated tertiary C(sp3)–H bonds). It has been successfully applied to the high incorporation of deuterium in 38 feedstock chemicals, 15 pharmaceutical compounds, and 6 drug precursors. Sequential deuteration between formyl C–H bonds of aldehydes and other activated hydridic C(sp3)–H bonds can be achieved in a selective manner.


 Please wait while we load your content...
Please wait while we load your content...