The crystal engineering of radiation-sensitive diacetylene cocrystals and salts†
Abstract
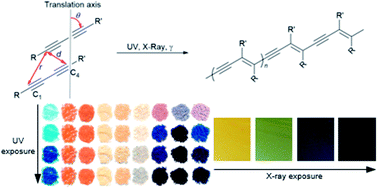
In this work we develop photoreactive cocrystals/salts of a commercially-important diacetylene, 10,12-pentacosadiynoic acid (PCDA, 1) and report the first X-ray crystal structures of PCDA based systems. The topochemical reactivity of the system is modified depending on the coformer used and correlates with the structural parameters. Crystallisation of 1 with 4,4′-azopyridine (2), 4,4′-bipyridyl (3), and trans-1,2-bis(4-pyridyl)ethylene (4) results in unreactive 2 : 1 cocrystals or a salt in the case of 4,4′-bipiperidine (5). However, salt formation with morpholine (6), diethylamine (7), and n-butylamine (8), results in highly photoreactive salts 12·7 and 1·8 whose reactivity can be explained using topochemical criteria. The salt 1·6 is also highly photoreactive and is compared to a model morpholinium butanoate salt. Resonance Raman spectroscopy reveals structural details of the photopolymer including its conformational disorder in comparison to less photoactive alkali metal salts and the extent of solid state conversion can be monitored by CP-MAS NMR spectroscopy. We also report an unusual catalysis in which amine evaporation from photopolymerised PCDA ammonium salts effectively acts as a catalyst for polymerisation of PCDA itself. The new photoreactive salts exhibit more reactivity but decreased conjugation compared to the commercial lithium salt and are of considerable practical potential in terms of tunable colours and greater range in UV, X-ray, and γ-ray dosimetry applications.


 Please wait while we load your content...
Please wait while we load your content...