Au3-to-Ag3 coordinate-covalent bonding and other supramolecular interactions with covalent bonding strength†
Abstract
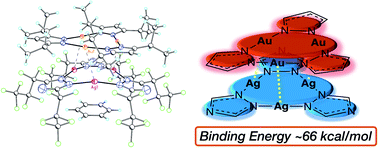
An efficient strategy for designing charge-transfer complexes using coinage metal cyclic trinuclear complexes (CTCs) is described herein. Due to opposite quadrupolar electrostatic contributions from metal ions and ligand substituents, [Au(μ-Pz-(i-C3H7)2)]3·[Ag(μ-Tz-(n-C3F7)2)]3 (Pz = pyrazolate, Tz = triazolate) has been obtained and its structure verified by single crystal X-ray diffraction – representing the 1st crystallographically-verified 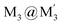 stacked adduct of monovalent coinage metal CTCs. Abundant supramolecular interactions with aggregate covalent bonding strength arise from a combination of M–M′ (Au → Ag), metal–π, π–π interactions and hydrogen bonding in this charge-transfer complex, according to density functional theory analyses, yielding a computed binding energy of 66 kcal mol−1 between the two trimer moieties – a large value for intermolecular interactions between adjacent d10 centres (nearly doubling the value for a recently-claimed Au(I) → Cu(I) polar-covalent bond: Proc. Natl. Acad. Sci. U.S.A., 2017, 114, E5042) – which becomes 87 kcal mol−1 with benzene stacking. Surprisingly, DFT analysis suggests that: (a) some other literature precedents should have attained a stacked
stacked adduct of monovalent coinage metal CTCs. Abundant supramolecular interactions with aggregate covalent bonding strength arise from a combination of M–M′ (Au → Ag), metal–π, π–π interactions and hydrogen bonding in this charge-transfer complex, according to density functional theory analyses, yielding a computed binding energy of 66 kcal mol−1 between the two trimer moieties – a large value for intermolecular interactions between adjacent d10 centres (nearly doubling the value for a recently-claimed Au(I) → Cu(I) polar-covalent bond: Proc. Natl. Acad. Sci. U.S.A., 2017, 114, E5042) – which becomes 87 kcal mol−1 with benzene stacking. Surprisingly, DFT analysis suggests that: (a) some other literature precedents should have attained a stacked 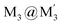 product akin to the one herein, with similar or even higher binding energy; and (b) a high overall intertrimer bonding energy by inferior electrostatic assistance, underscoring genuine orbital overlap between M and M′ frontier molecular orbitals in such polar-covalent M–M′ bonds in this family of molecules. The Au → Ag bonding is reminiscent of classical Werner-type coordinate-covalent bonds such as H3N: → Ag in [Ag(NH3)2]+, as demonstrated herein quantitatively. Solid-state and molecular modeling illustrate electron flow from the π-basic gold trimer to the π-acidic silver trimer with augmented contributions from ligand-to-ligand’ (LL′CT) and metal-to-ligand (MLCT) charge transfer.
product akin to the one herein, with similar or even higher binding energy; and (b) a high overall intertrimer bonding energy by inferior electrostatic assistance, underscoring genuine orbital overlap between M and M′ frontier molecular orbitals in such polar-covalent M–M′ bonds in this family of molecules. The Au → Ag bonding is reminiscent of classical Werner-type coordinate-covalent bonds such as H3N: → Ag in [Ag(NH3)2]+, as demonstrated herein quantitatively. Solid-state and molecular modeling illustrate electron flow from the π-basic gold trimer to the π-acidic silver trimer with augmented contributions from ligand-to-ligand’ (LL′CT) and metal-to-ligand (MLCT) charge transfer.


 Please wait while we load your content...
Please wait while we load your content...