N- and O-arylation of pyridin-2-ones with diaryliodonium salts: base-dependent orthogonal selectivity under metal-free conditions†
Abstract
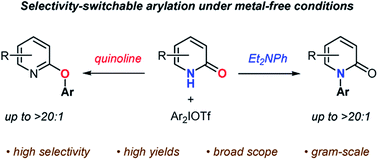
Metal-free N- and O-arylation reactions of pyridin-2-ones as ambident nucleophiles have been achieved with diaryliodonium salts on the basis of base-dependent chemoselectivity. In the presence of N,N-diethylaniline in fluorobenzene, pyridin-2-ones were very selectively converted to N-arylated products in high yields. On the other hand, the O-arylation reactions smoothly proceeded with the use of quinoline in chlorobenzene, leading to high yields and selectivities. In these methods, a variety of pyridin-2-ones in addition to pyridin-4-one and a set of diaryliodonium salts were accepted as suitable reaction partners.


 Please wait while we load your content...
Please wait while we load your content...