Pyrazolone ligation-mediated versatile sequential bioconjugations†
Abstract
Bioconjugation chemistries are critical tools in biotherapeutics discovery. The past efforts have been exclusively focused on two-segment conjugations. However, emerging research directions, such as polypharmacy biotherapeutics, desire multiple-component bioconjugations where more than two pharmacologically related biomolecules can be assembled into a single construct in high efficiency. We present here a set of sequential bioconjugation chemistries centered on a pyrazolone structural motif. It starts with a clickable “pyrazolone ligation” between a hydrazine group and a β-ketoester moiety followed by the conjugation between the newly formed pyrazolone core and an aldehyde-bearing biomolecule through a Knoevenagel reaction forming a Michael addition acceptor that can effectively capture a thiol-bearing biomolecule. When utilized intermolecularly, it quickly assembles four segments together forming a quadruple functional construct. When applied intramolecularly, it offers a set of highly diverse biomolecule scaffolds including stapled peptides and poly-macrocyclic peptides. We envision broad utilities of such sequential ligation chemistries.
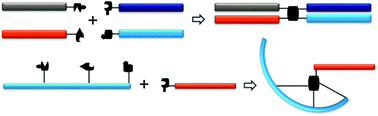


 Please wait while we load your content...
Please wait while we load your content...