Catalytic enantioselective synthesis of carbocyclic and heterocyclic spiranes via a decarboxylative aldol cyclization†
Abstract
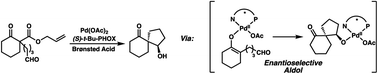
The synthesis of a variety of enantioenriched 1,3-diketospiranes from the corresponding racemic allyl β-ketoesters via an interrupted asymmetric allylic alkylation is disclosed. Substrates possessing pendant aldehydes undergo decarboxylative enolate formation in the presence of a chiral Pd catalyst and subsequently participate in an enantio- and diastereoselective, intramolecular aldol reaction to furnish spirocyclic β-hydroxy ketones which may be oxidized to the corresponding enantioenriched diketospiranes. Additionally, this chemistry has been extended to α-allylcarboxy lactam substrates leading to a formal synthesis of the natural product (−)-isonitramine.


 Please wait while we load your content...
Please wait while we load your content...