Regiocontrol in the oxidative Heck reaction of indole by ligand-enabled switch of the regioselectivity-determining step†
Abstract
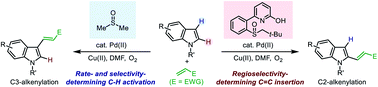
Efficient control of regioselectivity is a key concern in transition-metal-catalyzed direct C–H functionalization reactions. Various strategies for regiocontrol have been established by tuning the selectivity of the C–H activation step as a common mode. Herein, we present our study on an alternative mode of regiocontrol, in which the selectivity of the C–H activation step is no longer a key concern. We found that, in a reaction where the C–H activation step exhibits a different regio-preference from the subsequent functionalization step, a ligand-enabled switch of the regioselectivity-determining step could provide efficient regiocontrol. This mode has been exemplified by the Pd(II)-catalyzed aerobic oxidative Heck reaction of indoles, in which a ligand-controlled C3-/C2-selectivity was achieved for the first time by the development of sulfoxide-2-hydroxypyridine (SOHP) ligands.


 Please wait while we load your content...
Please wait while we load your content...