Multiple reactivities of flavonoids towards pathological elements in Alzheimer's disease: structure–activity relationship†
Abstract
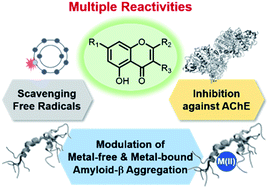
Amyloid-β (Aβ) accumulation, metal ion dyshomeostasis, oxidative stress, and cholinergic deficit are four major characteristics of Alzheimer's disease (AD). Herein, we report the reactivities of 12 flavonoids against four pathogenic elements of AD: metal-free and metal-bound Aβ, free radicals, and acetylcholinesterase. A series of 12 flavonoids was selected based on the molecular structures that are responsible for multiple reactivities including hydroxyl substitution and transfer of the B ring from C2 to C3. Our experimental and computational studies reveal that the catechol moiety, the hydroxyl groups at C3 and C7, and the position of the B ring are important for instilling multiple functions in flavonoids. We establish a structure–activity relationship of flavonoids that should be useful for designing chemical reagents with multiple reactivities against the pathological factors of AD.


 Please wait while we load your content...
Please wait while we load your content...