Photo-crosslink analysis in nonribosomal peptide synthetases reveals aberrant gel migration of branched crosslink isomers and spatial proximity between non-neighboring domains†
Abstract
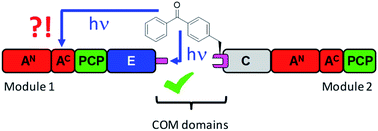
Nonribosomal peptide synthetases (NRPSs) are large, multi-modular enzyme templates for the biosynthesis of important peptide natural products. Modules are composed of a set of semi-autonomous domains that facilitate the individual reaction steps. Only little is known about the existence and relevance of a higher-order architecture in these mega-enzymes, for which contacts between non-neighboring domains in three-dimensional space would be characteristic. Similarly poorly understood is the structure of communication-mediating (COM) domains that facilitate NRPS subunit docking at the boundaries between epimerization and condensation domains. We investigated a COM domain pair in a minimal two module NRPS using genetically encoded photo-crosslinking moieties in the N-terminal acceptor COM domain. Crosslinks into the C-terminal donor COM domain of the partner module resulted in protein products with the expected migration behavior on SDS-PAGE gels corresponding to the added molecular weight of the proteins. Additionally, an unexpected apparent high-molecular weight crosslink product was revealed by mass spectrometric analysis to represent a T-form isomer with branched connectivity of the two polypeptide chains. Synthesis of the linear L-form and branched T-form isomers by click chemistry confirmed this designation. Our data revealed a surprising spatial proximity between the acceptor COM domain and the functionally unrelated small subdomain of the preceding adenylation domain. These findings provide an insight into three-dimensional domain arrangements in NRPSs in solution and suggest the described photo-crosslinking approach as a promising tool for the systematic investigation of their higher-order architecture.


 Please wait while we load your content...
Please wait while we load your content...