Palladium-catalysed 5-endo-trig allylic (hetero)arylation†
Abstract
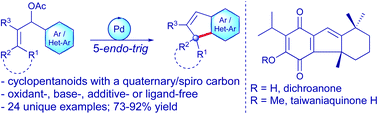
A palladium-catalysed intramolecular allylic (hetero)arylation strategy for the synthesis of fused cyclopentenes incorporated with all-carbon quaternary and spiro centres is described. The method is straightforward, shows broad scope, proceeds in synthetically useful yields, and provides a rare means to construct complex cyclopentanoids. The reaction is believed to involve a kinetically unfavourable 5-endo-trig carbocyclisation of the tethered (π-allyl)palladium system. Further, this method was successfully applied as the key step in the total synthesis of diterpene natural products taiwaniaquinone H and dichroanone.


 Please wait while we load your content...
Please wait while we load your content...