Photoinduced transition-metal- and external-photosensitizer-free intramolecular aryl rearrangement via C(Ar)–O bond cleavage†
Abstract
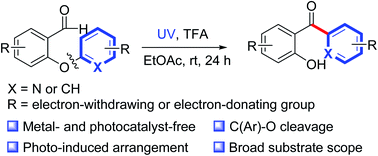
The use of photochemical reactions that do not require expensive photocatalysts or transition metals is an environmentally friendly strategy for accomplishing a variety of structural transformations. Herein, we report a protocol for photoinduced transition-metal- and external-photocatalyst-free intramolecular heteroaryl/aryl rearrangement reactions of 2-heteroaryl/aryloxybenzaldehydes. The protocol was compatible with a variety of functionalities, including methyl, methoxy, cyano, ester, trifluoromethyl, halogen, and heteroaromatic rings. Control experiments suggested that the reaction proceeded via a photoinduced intramolecular heteroaryl/aryl rearrangement process involving photoexcitation of the aldehyde carbonyl group, radical addition, C–C bond formation and C(Ar)–O bond cleavage.


 Please wait while we load your content...
Please wait while we load your content...