Highly efficient H2S scavengers via thiolysis of positively-charged NBD amines†
Abstract
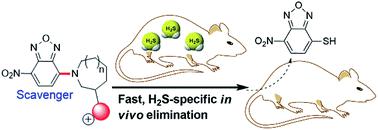
H2S is a well-known toxic gas and also a gaseous signaling molecule involved in many biological processes. Advanced chemical tools that can regulate H2S levels in vivo are useful for understanding H2S biology as well as its potential therapeutic effects. To this end, we have developed a series of 7-nitro-1,2,3-benzoxadiazole (NBD) amines as potential H2S scavengers. The kinetic studies of thiolysis reactions revealed that incorporation of positively-charged groups onto the NBD amines greatly increased the rate of the H2S-specific thiolysis reaction. We demonstrate that these reactions proceed effectively, with second order rate constants (k2) of >116 M−1 s−1 at 37 °C for NBD-S8. Additionally, we demonstrate that NBD-S8 can effectively scavenge enzymatically-produced and endogenous H2S in live cells. Furthering the biological significance, we demonstrate NBD-S8 mediates scavenging of H2S in mice.
- This article is part of the themed collection: Celebrating 100 Years of Chemistry at Nankai University


 Please wait while we load your content...
Please wait while we load your content...