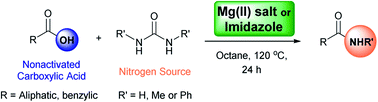
Direct synthesis of amides from nonactivated carboxylic acids using urea as nitrogen source and Mg(NO3)2 or imidazole as catalysts†‡
Abstract
A new method for the direct synthesis of primary and secondary amides from carboxylic acids is described using Mg(NO3)2·6H2O or imidazole as a low-cost and readily available catalyst, and urea as a stable, and easy to manipulate nitrogen source. This methodology is particularly useful for the direct synthesis of primary and methyl amides avoiding the use of ammonia and methylamine gas which can be tedious to manipulate. Furthermore, the transformation does not require the employment of coupling or activating agents which are commonly required.


 Please wait while we load your content...
Please wait while we load your content...