Cationic indium catalysts for ring opening polymerization: tuning reactivity with hemilabile ligands†
Abstract
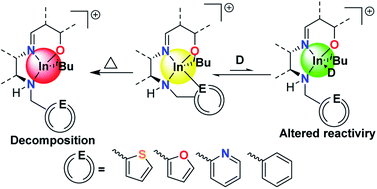
This is a comprehensive study of the effects of rationally designed hemilabile ligands on the stability, reactivity, and change in catalytic behavior of indium complexes. We report cationic alkyl indium complexes supported by a family of hemi-salen type ligands bearing hemilabile thiophenyl (2a), furfuryl (2b) and pyridyl (2c) pendant donor arms. Shelf-life and stability of these complexes followed the trend 2a < 2b < 2c, showing direct correlation to the affinity of the pendant donor group to the indium center. Reactivity towards polymerization of epichlorohydrin and cyclohexene oxide followed the trend 2a > 2b > 2c with control of polymerization following an inverse relationship to reactivity. Surprisingly, 2c polymerized racemic lactide without an external initiator, likely through an alkyl-initiated coordination-insertion mechanism.
- This article is part of the themed collection: Celebrating 10 years of Chemical Science


 Please wait while we load your content...
Please wait while we load your content...