Mildly regulated intrinsic faradaic layer at the oxide/water interface for improved photoelectrochemical performance†
Abstract
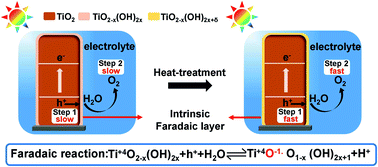
Metal oxides are widely used in different fields, including photoelectrocatalysis, photocatalysis, dye-sensitized solar cells, photoinduced superhydrophilicity and so on. It is well-known that there are intrinsic hydrated layers on the surfaces of metal oxides in ambient air or the electrolyte. Generally, interface layers between metal oxides and solutions have significant effects on the performances in these applications. However, the exact roles of the intrinsic hydrated layers are still unclear. In this study, taking TiO2 and Fe2O3 as model materials, we propose a mild heat treatment to increase the hydroxyl concentration in the hydrated surface layers of the oxides, which improves their photoelectrochemical performance remarkably. Moreover, we find that the heat-regulated hydrated layer plays the role of a hole transfer mediator between oxides and the electrolyte, which can accelerate both interface charge collection and oxygen evolution reaction kinetics in acidic solution. The new insights into the intrinsic hydrated interface layer on oxides can offer guidance not only in photoelectrocatalysis, but also in the other applications mentioned above.


 Please wait while we load your content...
Please wait while we load your content...