Redox deracemization of β,γ-alkynyl α-amino esters†
Abstract
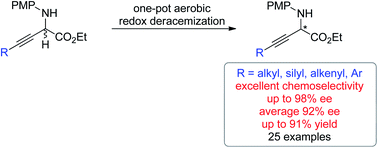
The first non-enzymatic redox deracemization method using molecular oxygen as the terminal oxidant has been described. The one-pot deracemization of β,γ-alkynyl α-amino esters consisted of a copper-catalyzed aerobic oxidation and chiral phosphoric acid-catalyzed asymmetric transfer hydrogenation with excellent functional group compatibility. By using benzothiazoline as the reducing reagent, an exclusive chemoselectivity at the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond over the C
N bond over the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond was achieved, allowing for efficient deracemization of a series of α-amino esters bearing diverse α-alkynyl substituent patterns. The origins of chemo- and enantio-selectivities were elucidated by experimental and computational mechanistic investigation. The generality of the strategy is further demonstrated by efficient deracemization of β,γ-alkenyl α-amino esters.
C bond was achieved, allowing for efficient deracemization of a series of α-amino esters bearing diverse α-alkynyl substituent patterns. The origins of chemo- and enantio-selectivities were elucidated by experimental and computational mechanistic investigation. The generality of the strategy is further demonstrated by efficient deracemization of β,γ-alkenyl α-amino esters.


 Please wait while we load your content...
Please wait while we load your content...