Allylic C(sp3)–H alkylation via synergistic organo- and photoredox catalyzed radical addition to imines†
Abstract
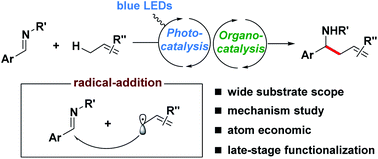
A new catalytic method for the direct alkylation of allylic C(sp3)–H bonds from unactivated alkenes via synergistic organo- and photoredox catalysis is described. The transformation achieves an efficient, redox-neutral synthesis of homoallylamines with broad functional group tolerance, under very mild reaction conditions. Mechanistic investigations indicate that the reaction proceeds through the N-centered radical intermediate which is generated by the allylic radical addition to the imine.
- This article is part of the themed collections: 2021 Nobel Prize in Chemistry – Asymmetric Organocatalysis and Most popular 2019-2020 organic chemistry articles


 Please wait while we load your content...
Please wait while we load your content...