A step-economic and one-pot access to chiral Cα-tetrasubstituted α-amino acid derivatives via a bicyclic imidazole-catalyzed direct enantioselective C-acylation†
Abstract
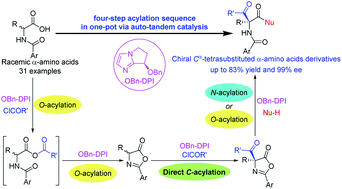
Cα-Tetrasubstituted α-amino acids are ubiquitous and unique structural units in bioactive natural products and pharmaceutical compounds. The asymmetric synthesis of these molecules has attracted a lot of attention, but a more efficient method is still greatly desired. Here we describe the first sequential four-step acylation reaction for the efficient synthesis of chiral Cα-tetrasubstituted α-amino acid derivatives from simple N-acylated amino acids via an auto-tandem catalysis using a single nucleophilic catalyst. The synthetic efficiency is improved via a direct enantioselective C-acylation; the methodology affords the corresponding Cα-tetrasubstituted α-amino acid derivatives with excellent enantioselectivities (up to 99% ee). This step-economic, one-pot, and auto-tandem strategy provides facile access to important chiral building blocks, such as peptides, serines, and oxazolines, which are often used in medicinal and synthetic chemistry.


 Please wait while we load your content...
Please wait while we load your content...