Enantioselective aerobic oxidative cross-dehydrogenative coupling of glycine derivatives with ketones and aldehydes via cooperative photoredox catalysis and organocatalysis†
Abstract
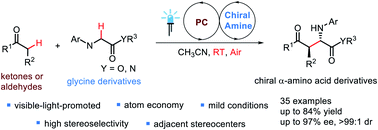
The combination of photoredox catalysis and enamine catalysis has enabled the development of an enantioselective aerobic oxidative cross-dehydrogenative coupling between glycine derivatives and simple ketones or aldehydes, which provides an efficient approach for the rapid synthesis of enantiopure unnatural α-alkyl α-amino acid derivatives in good yield with excellent diastereo- (up to >99 : 1) and enantioselectivities (up to 97% ee). This process includes the direct photoinduced oxidation of glycine derivatives to an imine intermediate, followed by the asymmetric Mannich-type reaction with an enamine intermediate generated in situ from a ketone or aldehyde and a chiral secondary amine organocatalyst. This mild method allows the direct formation of a C–C bond with simultaneous installation of two new stereocenters without wasteful removal of functional groups.
- This article is part of the themed collection: 2021 Nobel Prize in Chemistry – Asymmetric Organocatalysis


 Please wait while we load your content...
Please wait while we load your content...