H-bond donor-directed switching of diastereoselectivity in the Michael addition of α-azido ketones to nitroolefins†
Abstract
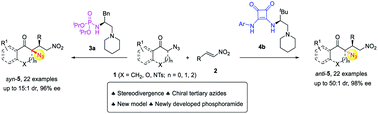
The development of catalyst-controlled stereodivergent asymmetric catalysis is important for providing facile access to all stereoisomers of chiral products with multiple stereocenters from the same starting materials. Despite progress, new design strategies for diastereodivergent asymmetric catalysis are still highly desirable. Here we report the potency of H-bond donors as the governing factor to tune diastereoselectivity in a highly diastereoselective switchable enantioselective Michael addition of α-azido ketones to nitroolefins. While a newly developed bifunctional tertiary amine, phosphoramide, preferentially afforded syn-adducts, an analogous squaramide catalyst selectively gave anti-adducts. The resulting multifunctional tertiary azides can be converted to spiro-pyrrolidines with four continuous stereocenters in a one-pot operation. Mechanistic studies cast light on the control of diastereoselectivity by H-bond donors. While the squaramide-catalyzed reaction proceeded with a transition state with both squaramide N–H bonds binding to an enolate intermediate, an unprecedented model was proposed for the phosphoramide-mediated reaction wherein an amide N–H bond and an alkylammonium ion formed in situ interact with nitroolefins, with the enolate stabilized by nonclassical C–H⋯O hydrogen-bonding interactions.


 Please wait while we load your content...
Please wait while we load your content...