High-efficiency dynamic sensing of biothiols in cancer cells with a fluorescent β-cyclodextrin supramolecular assembly†
Abstract
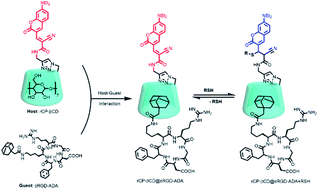
A unique fluorescent supramolecular assembly was constructed using coumarin-modified β-cyclodextrin as a reversible ratiometric probe and an adamantane-modified cyclic arginine–glycine–aspartate peptide as a cancer-targeting agent via host–guest inclusion complexation. Importantly, the coumarin-modified β-cyclodextrin not only showed higher sensitivity than the parent coumarin derivatives owing to the presence of numerous hydroxyl groups on the cyclodextrin but also provided a hydrophobic cavity for encapsulation of a cancer-targeting agent. The assembly showed a reversible and fast response to biothiols with a micromolar dissociation constant, as well as outstanding cancer cell permeability, which can be used for high-efficiency real-time monitoring of biothiols in cancer cells. This supramolecular assembly strategy endows the fluorescent probe with superior performance for dynamic sensing of biothiols.
- This article is part of the themed collection: Celebrating 100 Years of Chemistry at Nankai University


 Please wait while we load your content...
Please wait while we load your content...