Construction of heterometallic clusters with multiple uranium–metal bonds by using dianionic nitrogen–phosphorus ligands†
Abstract
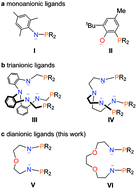
Compared with the prevalent metal–metal bond in transition metals, examples of the actinide–metal bond in heterometallic clusters are rare. Herein, a series of heterometallic clusters with multiple uranium–metal bonds has been prepared based on two newly synthesized nitrogen–phosphorus ligands L1 {O[(CH2)2NHP(iPr)2]2} and L2 {[CH2O(CH2)2NHP(iPr)2]2}. Different P–P distances, 6.069 and 4.464 Å, are observed in the corresponding uranium complexes 1 {O[(CH2)2NP(iPr)2]2UCl2} and 2 {[CH2O(CH2)2NP(iPr)2]2UCl2}, respectively, and lead to the different coordination modes with transition metals. The reactions of zero-valent group 10 metal compounds with complex 1 generate heterometallic clusters (3-U2Ni2 and 4-U2Pd2) featuring four uranium–metal bonds; whereas reactions with 2 afford one-dimensional metal-chain 5-(UNi)n, bimetallic species 6-UPd, and a tri-platinum bridged diuranium molecular cluster 7-U2Pt3. Complex 5-(UNi)n represents the first infinite chain containing the U–M bond and 7-U2Pt3 is the first species with multiple U–Pt bonds. This study further highlights the important role of ligands in the construction of multiple uranium–metal bonds and may allow the synthesis of novel d–f heterometallic clusters and the investigation of their applications in catalysis and small-molecule activation.
- This article is part of the themed collections: Celebrating 10 years of Chemical Science and Celebrating a century of chemical excellence at Nanjing University


 Please wait while we load your content...
Please wait while we load your content...