Tuning the anion binding properties of lanthanide receptors to discriminate nucleoside phosphates in a sensing array†
Abstract
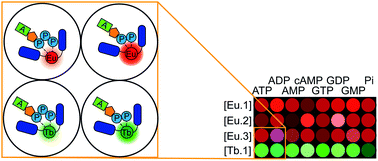
The development of synthetic receptors for the selective binding and discrimination of anions in water requires an understanding of how anions interact with these synthetic receptors. Molecules designed to differentiate nucleoside phosphate anions (e.g. ATP, ADP, GTP, GDP, UDP) under physiological conditions could underpin exciting new sensing tools for biomedical research and drug discovery, but it is very challenging due to the similarities in anion structure, size and charge. We present a series of lanthanide-based anion receptors and establish key structural elements that impact on nucleoside phosphate anion binding and sensing. Structural evidence of anion binding using X-ray crystallographic and NMR data, supported by DFT calculations indicate the binding modes between the lanthanide complexes and certain phosphoanions, revealing a bidentate (α-, γ-) binding mode to ATP. We further use four of the receptors to allow discrimination of eight nucleoside phosphate anions in the first array-based assay using lanthanide complexes, taking advantage of the multiple emission bands and long emission lifetimes associated with luminescent lanthanide complexes.
- This article is part of the themed collections: 2020 Chemical Science HOT Article Collection and The Mechanics of Supramolecular Chemistry


 Please wait while we load your content...
Please wait while we load your content...