meta-Selective C–H functionalisation of aryl boronic acids directed by a MIDA-derived boronate ester†
Abstract
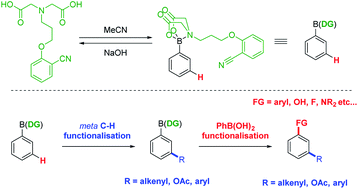
N-Methyliminodiacetic acid (MIDA) boronates are boronic acid derivatives which are stable to reduction, oxidation and transmetalation. This has led to their widespread use as boronic acid protecting groups (PGs) and in iterative cross-couplings. We describe herein the development of a novel MIDA derivative that acts in a dual manner, as a protecting group and a directing group (DG) for meta C(sp2)–H functionalisation of arylboronic acids. Palladium catalysed C–H alkenylations, acetoxylations and arylations are possible, at room temperature and under aerobic conditions. Deprotection to reveal the functionalised boronic acids is rapid and allows for full recovery of the DG. The technique allows the facile diversification of aryl boronic acids and their subsequent use in a range of reactions or in iterative processes.


 Please wait while we load your content...
Please wait while we load your content...