Synthesis of unsymmetrically substituted triarylamines via acceptorless dehydrogenative aromatization using a Pd/C and p-toluenesulfonic acid hybrid relay catalyst†
Abstract
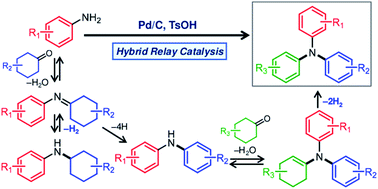
An efficient and convenient procedure for synthesizing triarylamines based on a dehydrogenative aromatization strategy has been developed. A hybrid relay catalyst comprising carbon-supported Pd (Pd/C) and p-toluenesulfonic acid (TsOH) was found to be effective for synthesizing a variety of triarylamines bearing different aryl groups starting from arylamines (diarylamines or anilines), using cyclohexanones as the arylation sources under acceptorless conditions with the release of gaseous H2. The proposed reaction comprises the following relay steps: condensation of arylamines and cyclohexanones to produce imines or enamines, dehydrogenative aromatization of the imines or enamines over Pd nanoparticles (NPs), and elimination of H2 from the Pd NPs. In this study, an interesting finding was obtained indicating that TsOH may promote the dehydrogenation.


 Please wait while we load your content...
Please wait while we load your content...