Catalytic enantioselective arylative cyclizations of alkynyl 1,3-diketones by 1,4-rhodium(i) migration†
Abstract
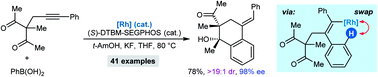
The enantioselective synthesis of densely functionalized polycarbocycles by the rhodium(I)-catalyzed reaction of arylboronic acids with 1,3-diketones is described. The key step in these desymmetrizing domino addition–cyclization reactions is an alkenyl-to-aryl 1,4-Rh(I) migration, which enables arylboronic acids to function effectively as 1,2-dimetalloarene surrogates.


 Please wait while we load your content...
Please wait while we load your content...