Diazaphosphinanes as hydride, hydrogen atom, proton or electron donors under transition-metal-free conditions: thermodynamics, kinetics, and synthetic applications†
Abstract
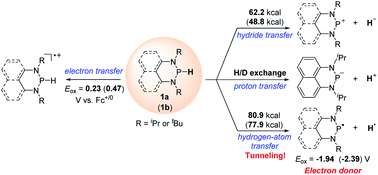
Exploration of new hydrogen donors is in large demand in hydrogenation chemistry. Herein, we developed a new 1,3,2-diazaphosphinane 1a, which can serve as a hydride, hydrogen atom or proton donor without transition-metal mediation. The thermodynamics and kinetics of these three pathways of 1a, together with those of its analog 1b, were investigated in acetonitrile. It is noteworthy that, the reduction potentials (Ered) of the phosphenium cations 1a-[P]+ and 1b-[P]+ are extremely low, being −1.94 and −2.39 V (vs. Fc+/0), respectively, enabling corresponding phosphinyl radicals to function as neutral super-electron-donors. Kinetic studies revealed an extraordinarily large kinetic isotope effect KIE(1a) of 31.3 for the hydrogen atom transfer from 1a to the 2,4,6-tri-(tert-butyl)-phenoxyl radical, implying a tunneling effect. Furthermore, successful applications of these diverse P–H bond energetic parameters in organic syntheses were exemplified, shedding light on more exploitations of these versatile and powerful diazaphosphinane reagents in organic chemistry.
- This article is part of the themed collection: Celebrating 100 Years of Chemistry at Nankai University


 Please wait while we load your content...
Please wait while we load your content...