Tipping the balance: theoretical interrogation of divergent extended heterolytic fragmentations†
Abstract
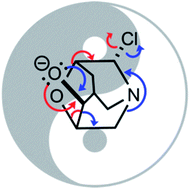
Herein we interrogate a type of heterolytic fragmentation reaction called a ‘divergent fragmentation’ using density functional theory (DFT), natural bond orbital (NBO) analysis, ab initio molecular dynamics (AIMD), and external electric field (EEF) calculations. We demonstrate that substituents, electrostatic environment and non-statistical dynamic effects all influence product selectivity in reactions that involve divergent fragmentation pathways. Direct dynamics simulations reveal an unexpected post-transition state bifurcation (PTSB), and EEF calculations suggest that some transition states for divergent pathways can, in principle, be selectively stabilized if an electric field of the correct magnitude is oriented appropriately.


 Please wait while we load your content...
Please wait while we load your content...