Photocatalytic carbanion generation from C–H bonds – reductant free Barbier/Grignard-type reactions†
Abstract
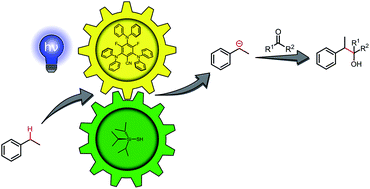
We report a redox-neutral method for the generation of carbanions from benzylic C–H bonds in a photocatalytic Grignard-type reaction. The combination of photo- and hydrogen atom transfer (HAT) catalysis enables the abstraction of a benzylic hydrogen atom, generating a radical intermediate. This radical is reduced in situ by the organic photocatalyst to a carbanion, which is able to react with electrophiles such as aldehydes or ketones, yielding homobenzylic secondary and tertiary alcohols.
- This article is part of the themed collection: Most popular 2018-2019 organic chemistry articles


 Please wait while we load your content...
Please wait while we load your content...