Photochemical ring expansion reactions: synthesis of tetrahydrofuran derivatives and mechanism studies†
Abstract
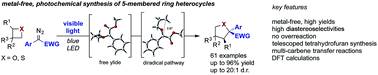
The reaction mechanism of oxygen and sulfur ylide mediated rearrangements is even today a matter of debate. In this report, we describe ring expansion reactions of oxetane and thietane heterocycles that allow probing the underlying reaction mechanism under metal-free, photochemical conditions. This ring expansion proves highly efficient and allows the synthesis of tetrahydrofuran and thiolane heterocycles under mild and operationally simple reaction conditions. These studies reveal marked differences in the stereoselectivity of the ring expansion of oxygen or sulfur ylides, which were further investigated computationally. DFT calculations show that carbenes react under ylide formation and that the corresponding ring expansion reactions proceed via a diradical pathway. The different bond lengths in free oxygen or sulfur ylide intermediates cause the distinctive stereochemical outcome.


 Please wait while we load your content...
Please wait while we load your content...