Sulphide as a leaving group: highly stereoselective bromination of alkyl phenyl sulphides†
Abstract
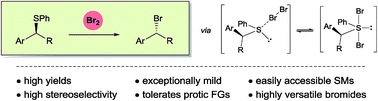
A conceptionally novel nucleophilic substitution approach to synthetically important alkyl bromides is presented. Using molecular bromine (Br2), readily available secondary benzyl and tertiary alkyl phenyl sulphides are converted into the corresponding bromides under exceptionally mild, acid- and base-free reaction conditions. This simple transformation allows the isolation of elimination sensitive benzylic β-bromo carbonyl and nitrile compounds in mostly high yields and purities. Remarkably, protic functionalities such as acids and alcohols are tolerated. Enantioenriched benzylic β-sulphido esters, readily prepared by asymmetric sulpha-Michael addition, produce the corresponding inverted bromides with high stereoselectivities, approaching complete enantiospecificity at −40 °C. Significantly, the reported benzylic β-bromo esters can be stored without racemisation for prolonged periods at −20 °C. Their synthetic potential was demonstrated by the one-pot preparation of γ-azido alcohol (S)-5 in 90% ee. NMR studies revealed an initial formation of a sulphide bromine adduct, which in turn is in equilibrium with a postulated dibromosulphurane intermediate that undergoes C–Br bond formation.


 Please wait while we load your content...
Please wait while we load your content...