Reactions of thermally generated benzynes with six-membered N-heteroaromatics: pathway and product diversity†
Abstract
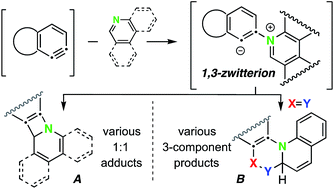
We report here various pathways by which six-membered N-heteroaromatic compounds react with benzynes that are generated by the HDDA reaction. The initially formed 1,3-zwitterionic species (a) can collapse intramolecularly to give novel 1 : 1 adducts of the heterocycle and benzyne; (b) can react with an externally added, electrophilic third-component to give functionalized heterocyclic products; or (c) can react with an external protic nucleophile to produce, following collapse of the ion pair resulting from protonation of the zwitterion, a variety of three-component assemblies. Mechanisms for formation of some of the 1 : 1 adducts are supported by DFT methods. The scope of the protic nucleophilic coupling was also expanded to a two-pot operation by using triflic acid as a protic “non-nucleophile”, followed by the addition of a suitably reactive nucleophile.


 Please wait while we load your content...
Please wait while we load your content...