A remarkably strained cyclopyrenylene trimer that undergoes metal-free direct oxygen insertion into the biaryl C–C σ-bond†
Abstract
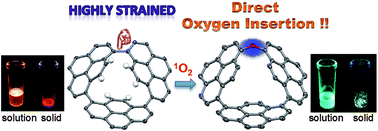
A remarkably strained cyclopyrenylene trimer CP3 was synthesized and it underwent the first biaryl C–C σ-bond cleavage by direct oxygen insertion without the aid of any metal agents. A priori highly strained CP3 exhibits the longest wavelength emission among all pyrene-based fluorophores due to the intensive electronic interactions between pyrenes. The color of the emission drastically changes from orange to light blue upon oxidation. Theoretical studies revealed that the release of ring strain reasonably drives the reaction between two CP3 molecules and O2. This strain-induced transformation could be also applied for sulfur atom insertion into a biaryl σ-bond.


 Please wait while we load your content...
Please wait while we load your content...