Desymmetrization of cyclic 1,3-diketones via Ir-catalyzed hydrogenation: an efficient approach to cyclic hydroxy ketones with a chiral quaternary carbon†
Abstract
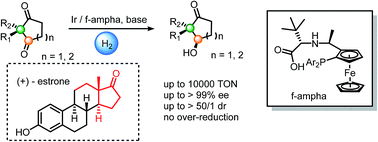
We herein report an efficient method to synthesize cyclic hydroxy ketones with a chiral quaternary center. Catalyzed by an Ir/f-ampha complex, cyclic α,α-disubstituted 1,3-diketones were hydrogenated, giving mono-reduced products with both high enantioselectivities and diastereoselectivities. In addition, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bonds could survive in this catalytic system. This method was applied in the preparation of (+)-estrone. No diols were observed in this chemical transformation. The enantiomeric and diastereomeric induction were achieved as a result of steric hindrance.
C bonds could survive in this catalytic system. This method was applied in the preparation of (+)-estrone. No diols were observed in this chemical transformation. The enantiomeric and diastereomeric induction were achieved as a result of steric hindrance.


 Please wait while we load your content...
Please wait while we load your content...