Site-selective C–H activation and regiospecific annulation using propargylic carbonates†
Abstract
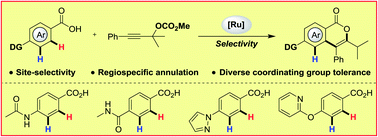
Herein we describe an unprecedented RuII-catalyzed site-selective and regiospecific annulation of benzoic acids with propargylic carbonates. The weakly coordinating carboxylic acid moiety outperformed other typically used directing groups in C–H activation, including ketone, nitrile, sulfonamide, amide and strongly coordinating nitrogen heterocycles. This is an important step towards the application of C–H activation reactions in complex (functional) real-world molecules.


 Please wait while we load your content...
Please wait while we load your content...