Ligand and counteranion enabled regiodivergent C–H bond functionalization of naphthols with α-aryl-α-diazoesters†
Abstract
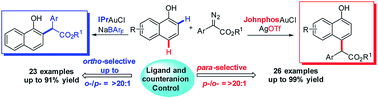
Here, an unprecedented ligand and counteranion-controlled and site-selectivity switchable direct C–H bond functionalization of unprotected naphthols with α-aryl-α-diazoesters was developed. In this transformation, site selectivities are realized by turning on/off the coordination between metal complexes and hydroxy groups. The preliminary mechanism revealed that the interaction between the hydroxy group and gold catalyst plays a key role in switching the site-selectivity of gold-carbene. This protocol potentially provides a novel design for C–H bond functionalization.


 Please wait while we load your content...
Please wait while we load your content...