A regioselectivity switch in Pd-catalyzed hydroallylation of alkynes†
Abstract
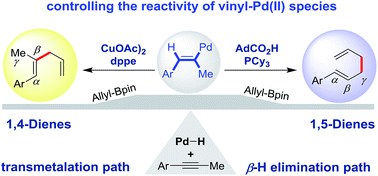
By exploiting the reactivity of a vinyl-Pd species, we control the regioselectivity in hydroallylation of alkynes under Pd-hydride catalysis. A monophosphine ligand and carboxylic acid combination promotes 1,5-dienes through a pathway involving isomerization of alkynes to allenes. In contrast, a bisphosphine ligand and copper cocatalyst favor 1,4-dienes via a mechanism that involves transmetalation. Our study highlights how to access different isomers by diverting a common organometallic intermediate.
- This article is part of the themed collection: New reactivity in organic chemistry


 Please wait while we load your content...
Please wait while we load your content...