Isomeric triazines exhibit unique profiles of bioorthogonal reactivity†
Abstract
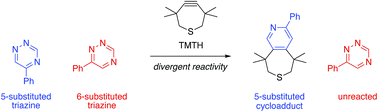
Expanding the scope of bioorthogonal reactivity requires access to new and mutually compatible reagents. We report here that 1,2,4-triazines can be tuned to exhibit unique reaction profiles with biocompatible strained alkenes and alkynes. Computational analyses were used to identify candidate orthogonal reactions, and the predictions were experimentally verified. Notably, 5-substituted triazines, unlike their 6-substituted counterparts, undergo rapid [4 + 2] cycloadditions with a sterically encumbered strained alkyne. This unique, sterically controlled reactivity was exploited for dual bioorthogonal labeling. Mutually orthogonal triazines and cycloaddition chemistries will enable new multi-component imaging applications.
- This article is part of the themed collections: Most popular 2019-2020 chemical biology articles and Most popular 2018-2019 chemical biology articles


 Please wait while we load your content...
Please wait while we load your content...