S8-Catalyzed triple cleavage of bromodifluoro compounds for the assembly of N-containing heterocycles†
Abstract
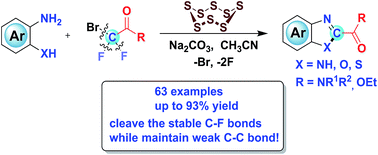
An unprecedented S8-catalyzed selective triple-cleavage of bromodifluoroacetamides is disclosed for the first time. Valuable 2-amido substituted benzimidazoles, benzoxazoles and benzothiazoles were obtained in good to excellent yields in a cascade protocol in this strategy. Mechanistic studies suggested that a C2 source was generated in situ by selective cleavage of three C–X bonds, including two inert C(sp3)–F bonds on bromodifluoroacetamides, while leaving C–C bonds intact. This strategy will undoubtedly further consummate the role of halo difluoro compounds and enrich both fluorine chemistry and pharmaceutical sciences.
- This article is part of the themed collection: Editor’s Choice – Ning Jiao


 Please wait while we load your content...
Please wait while we load your content...