Assembly of 1H-isoindole derivatives by selective carbon–nitrogen triple bond activation: access to aggregation-induced emission fluorophores for lipid droplet imaging†
Abstract
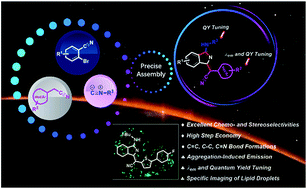
A novel strategy has been established to assemble a series of single (Z)- or (E)-1H-isoindole derivatives through selectively and sequentially activating carbon–nitrogen triple bonds in a multicomponent system containing various nucleophilic and electrophilic sites. The reaction provides efficient access to structurally unique fluorophores with aggregation-induced emission characteristics. These new fluorophores show fluorescence wavelengths and efficiencies that can be modulated and have excellent potential to specifically light up lipid droplets (LDs) in living cells with bright fluorescence, low cytotoxicity and better photostability than commercially available LD-specific dyes.


 Please wait while we load your content...
Please wait while we load your content...