Doubly zwitterionic, di-reduced, highly electron-rich, air-stable naphthalenediimides: redox-switchable islands of aromatic–antiaromatic states†
Abstract
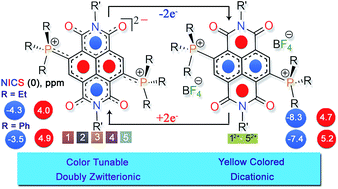
The di-reduced state of the naphthalene moiety and its congeners have long captivated chemists as it is elusive to stabilize these intrinsically reactive electron-rich π-systems and for their emergent multifaceted properties. Herein we report the synthesis and isolation of two-electron (2e−) reduced, highly electron-rich naphthalenediimides (NDIs). A doubly zwitterionic structure is observed for the first time in a naphthalene moiety and validated by single crystal X-ray crystallography and spectroscopic methods. The synthesis avoids hazardous reducing agents and offers an easy, high-yielding route to bench-stable di-reduced NDIs. Notably, we realized high negative first oxidation potentials of up to −0.730 V vs. Fc/Fc+ in these systems, which establish these systems to be one of the strongest ambient stable electron donors. The study also provides the first insights into the NMR spectra of the di-reduced systems revealing a large decrease in diatropicity of the naphthalene ring compared to its 2e− oxidized form. The NICS, NICS-XY global ring current, gauge-including magnetically induced current (GIMIC) and AICD ring current density calculations revealed switching of the antiaromatic and aromatic states at the naphthalene and the imide rings, respectively, in the di-reduced system compared to the 2e− oxidized form. Notably, the substituents at the phosphonium groups significantly tune the antiaromatic–aromatic states and donor ability, and bestow an array of colors to the di-reduced systems by virtue of intramolecular through-space communication with the NDI scaffold. Computational studies showed intramolecular noncovalent interactions to provide additional stability to these unprecedented doubly zwitterionic systems.


 Please wait while we load your content...
Please wait while we load your content...