A novel mass spectrometry-cleavable, phosphate-based enrichable and multi-targeting protein cross-linker†
Abstract
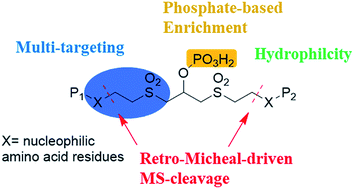
Chemical cross-linking mass spectrometry (XL-MS) is a powerful technology for obtaining protein structural information and studying protein–protein interactions. We report phospho-bisvinylsulfone (pBVS) as a novel water-soluble, MS-cleavable, phosphate-based enrichable and multi-targeting cross-linker. In this approach, the fragmentation of pBVS cross-linked peptides occurs in situ through retro-Michael addition. The phosphate group is successfully used as a small affinity tag to isolate cross-linked peptides from the highly abundant non-cross-linked peptides. In addition, the linker targets multiple types of amino acid residues, including cysteine, lysine and histidine. This method was applied to cross-link bovine serum albumin (BSA), myoglobin and Lbcpf1 demonstrating the ability to yield accurate and abundant information to facilitate protein structure elucidation.
- This article is part of the themed collection: 2019 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...