Trifluoromethylthiolation–arylation of diazocarbonyl compounds by modified Hooz multicomponent coupling†
Abstract
A new Zn-mediated trifluoromethylthiolation-based bifunctionalization reaction is developed. In this process, simultaneous C–SCF3 and C–C bond formation takes place in a multicomponent reaction, in which an aryl and a SCF3 group arise from different reagents. Our studies show that the reaction mechanism is similar to the Hooz multicomponent coupling. The process involves in situ generation of BAr3, which reacts with a diazocarbonyl compound, and the reaction is terminated by an electrophilic SCF3 transfer. The reaction can also be extended to fluorination based bifunctionalization which proceeds with somewhat lower yield than the analogous trifluoromethylthiolation reaction.
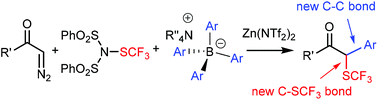
- This article is part of the themed collection: Most popular 2019-2020 organic chemistry articles


 Please wait while we load your content...
Please wait while we load your content...