Gold-catalyzed bicyclic annulations of 4-methoxy-1,2-dienyl-5-ynes with isoxazoles to form indolizine derivatives via an Au-π-allene intermediate†
Abstract
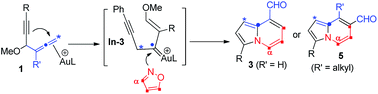
Gold-catalyzed bicyclic annulations of 4-methoxy-1,2-dienyl-5-ynes with isoxazoles afford indolizine derivatives with a structural rearrangement. The mechanism of these new annulations does not involve α-imino gold carbenes generated from gold π-alkyne intermediates. We postulate alkyne attack on gold π-allenes, yielding vinyl gold carbenes. These newly generated carbenes react with isoxazole derivatives to yield Z-3-imino-2-en-1-als, further enabling sequential cyclizations to deliver indolizine derivatives in two distinct classes.


 Please wait while we load your content...
Please wait while we load your content...