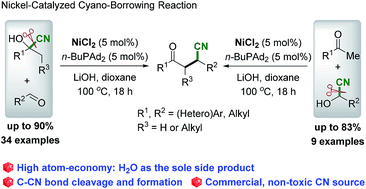
Cyano-borrowing reaction: nickel-catalyzed direct conversion of cyanohydrins and aldehydes/ketones to β-cyano ketone†
Abstract
A direct nickel-catalyzed, high atom- and step-economical reaction of cyanohydrins with aldehydes or ketones via an unprecedented “cyano-borrowing reaction” has been developed. Cleavage of the C–CN bond of cyanohydrins followed by aldol condensation and conjugate addition of cyanide to α,β-unsaturated ketones proceeded to deliver a range of racemic β-cyano ketones with good to high yields. The practical procedure with the use of a commercial and less-toxic CN source bodes well for wide application of this protocol.


 Please wait while we load your content...
Please wait while we load your content...