Brønsted acid and Pd–PHOX dual-catalysed enantioselective addition of activated C-pronucleophiles to internal dienes†
Abstract
We describe the development of Pd–PHOX-catalysed enantioselective couplings of internal dienes with malononitrile and other activated C-pronucleophiles. Reactions are dramatically accelerated by the addition of Et3N·HBArF4 as a Brønsted acid co-catalyst, enabling a suite of products bearing a variety of alkyl substituents at the stereogenic carbon to be prepared. A series of mechanism-oriented experiments reveal key aspects of the catalytic cycle and the importance of the non-coordinating BArF4 counterion in not only promoting reactions of internal dienes but also additions of previously unreactive nucleophiles towards terminal dienes.
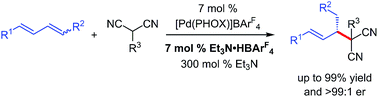


 Please wait while we load your content...
Please wait while we load your content...