A ruthenium(ii)-catalyzed C–H allenylation-based approach to allenoic acids†
Abstract
A Ru(II)-catalyzed direct access to various functionalized allenoic acids via C–H allenylation of readily available aryl carboxylic acids with propargylic acetates is reported. Axially chiral allenoic acids could be obtained in high ee by using optically active propargylic acetates through a chirality transfer strategy.
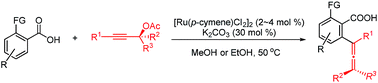


 Please wait while we load your content...
Please wait while we load your content...