Non-stabilized diazoalkane synthesis via the oxidation of free hydrazones by iodosylbenzene and application in in situ MIRC cyclopropanation†
Abstract
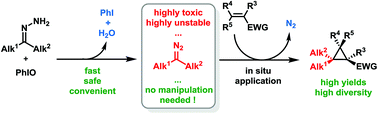
Electron-rich alkyl diazo compounds are powerful reagents in organic synthesis, but the risks associated with their toxicity and instability often limit their uses. Herein we describe an efficient, easy-to-handle and safe batch protocol for the in situ generation and cyclopropanation of these highly reactive non-stabilized diazoalkanes through the oxidation of free hydrazones using iodosylbenzene. Numerous substituted cyclopropanes have been synthesized using this methodology, including various gem-dimethylcyclopropanes of particular interest in medicinal chemistry.


 Please wait while we load your content...
Please wait while we load your content...